What Is the Steric Number of O3
- Molecules with a steric number of 3 are built on the trigonal planar electronic geometry. Acetylene C 2 H 2 - The carbons are bonded by a triple bond.
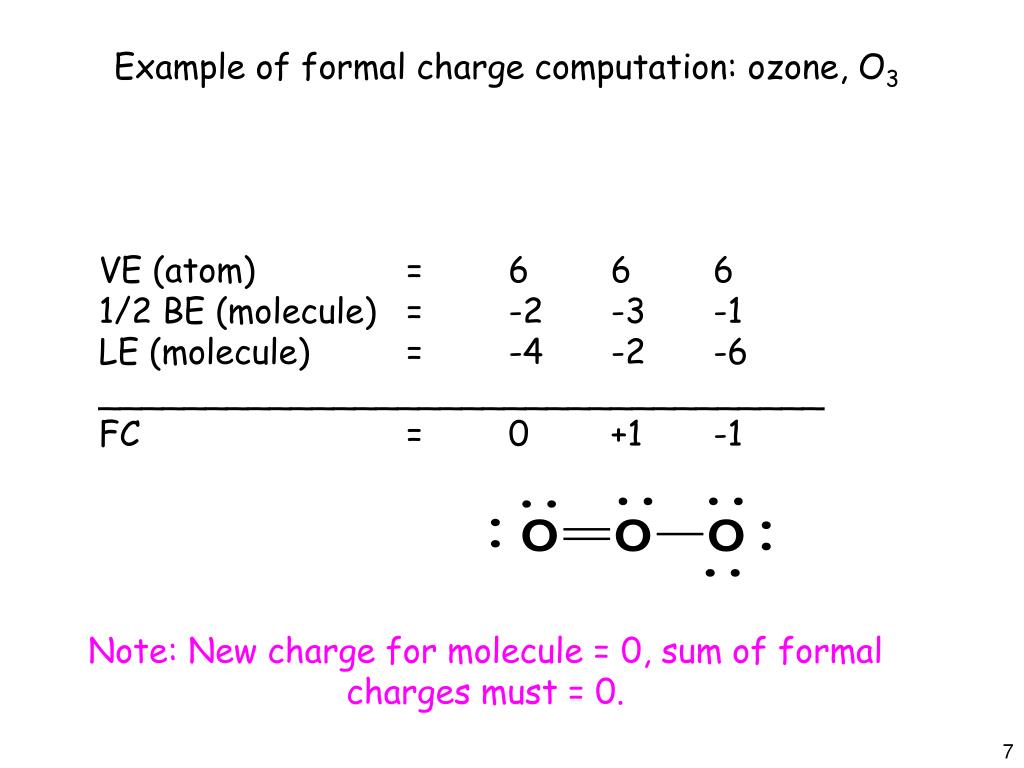
Ppt Lewis Structures And The Geometry Of Molecules With A Central Atom Powerpoint Presentation Id 6380170
Calculating X plus E nitrate has a steric number of 3.

. 1 lone pair Formula with a steric number of 4. The bond angles are approximately 120 in molecules with SN 3. O3 Valence electrons.
What is the steric number of O3Ozone Which statement correctly describes what happens to Li and Cl atoms when they bond. No lone pairs trigonal planar bond angle 120 eg SO3. In the case of CH3F molecules the number of sigma bonds is 4 one C-F bond and three C-H bonds while the number of lone pairs is zero.
Science Chemistry QA Library ClO3. As a result the atoms steric number will be equal to 4. Loses 1 valence electron and becomes a positive ion gains 1 valence electron and becomes a.
It will form two single bonds one with each hydrogen atom that account for 4 valence electrons and has 2 lone pairs of electrons that account for the other 4 valence electrons. Sign indicates gaining an electron. In Ozone or O3 there are six valence electrons for each molecule of Oxygen.
Steric Number Number of Bonded atoms plus Lone Pairs. Here as there are three oxygen molecules the total number of valence electrons is 63 18. Li loses 1 valence electron and becomes a negative ion Cl gai.
Ns 1 valence electron and becomes a positive ion. Geometrics atom NH3 CH4 BrF5 BrF3 CO32 CO2 H2O IC2- ICI4-Question. Summary of VSEPR Film and Lecture.
Determine the molecular geometry of CF2Cl2. - The bond angle is 180 in molecules with SN 2. IF7 can exist and FI7 can not exist why not what is the steric number for iodine atom in IF7.
Because nitrogen has no lone electron pairs E is equal to zero. Xenon is a noble gas element or inert gas element. S bond angle Hybrid orbital type.
Also used to bleach substances and killing microorganisms in air and water sources. 3 2 bonding sites. The atomic number of xenon is 54 and its electronic configuration is.
There are 2 bonded atoms and no lone pairs. We review their content and use your feedback to keep the quality high. The central nitrogen atom in nitrate has three X ligands due to the three bonded oxygen atoms.
Then calculate the steric number. The sum of X and E is the steric number. Steric number of XeO3 Number of bonded atoms attached to xenon Lone pair on xenon As per the lewis structure of XeO3 the xenon atom is bonded with three oxygen atoms and it contains 1 lone pair of electrons.
From the steric number determine the type of hybridization and geometry. - Molecules with a steric number of 2 are built on the linear electronic geometry. Experts are tested by Chegg as specialists in their subject area.
Carbon Dioxide CO 2 - Carbon dioxide is an example of a compound that contains 2 sets of double bonds. Steric Numbers Overall Electron Pair Hybridization of the central Formula Lewis Structure Bond Angles Shape Net Dipole. Please show me how to complete this chart.
The central atom is a xenon atom. Sign indicates losing an electron from the total valence electrons. These molecules have a linear molecular geometry shape.
Structures of Molecule Names. There are 2 oxygen atoms bonded to carbon with no lone pairs so the steric number is 2. Li loses 1 valence electron and becomes a negative ion Cl gai.
Calculate the steric number that is the sum of the number of lone pairs and sigma bonds. Thus there are a total of 18 valence electrons available for Ozone molecule. View the full answer.
As you can see the oxygen atom is surrounded by 4 regions of electron density 2 single bonds and 2 lone pairs of electrons. Steric number of XeO3 3 1 4. One lone pair bent bond angle.
What is the steric number of O3. Steric number of an atomfor example the N of NH4 within a compound is calculated by adding up the number of atoms bonded to that atomHs bonded to the N plus the number of lone electron pairs. What is the steric number of the central sulfur atom in SO3.
Hydrogen 1 3Hydrogen 3 Oxygen 6 Total 9. Steric number 3. The Central atom of formaldehyde is Carbon C here carb.
In the Stratosphere the ozone layer filters out UV radiation from the sun that would be harmful to life in large doses. What is the steric number of O3Ozone Which statement correctly describes what happens to Li and Cl atoms when they bond. Ns 1 valence electron and becomes a positive ion.
Now the important point is not to forget about the sign. Here the steric number comes out to be 4 which indicates sp3 hybridization that is tetrahedral molecular geometry. 4 109½ o sp3 hybrid orbitals 4 total orbitals 3 120o sp2 hybrid orbitals 3 total orbitals 2 180 sp hybrid orbitals 2 total orbitals 1 no angle s orbital Only hydrogen has a S of 1.
Loses 1 valence electron and becomes a positive ion gains 1 valence electron and becomes a. First of all we need to calculate the total number of valence electrons present in hydronium ion. Central atom Cl Type of hybrid orbitals on the chlorine atom _____ sp sp2 sp3 Number of hybrid orbitals used in overlap with atomic orbitals Number of hybrid orbitals accomodating unshared electrons Hybrid orbital geometry _____linear bent trigonal planar trigonal pyramidal tetrahedral Molecular geometry _____linear bent trigonal.
What is the steric number or the number of electron groups around the central atom of. SN 3 -- 2 bonded atoms. Steric number of an atomfor example the N of NH4 within a compound is calculated by.
Steric number 2.

O3 Lewis Structure Polarity Hybridization Shape And Molecular Geometry

1 Covalent Bonding Sharing Of Electron Pairs By Atoms Ppt Video Online Download

No comments for "What Is the Steric Number of O3"
Post a Comment